

Pd-Cu/C上甲酸根电催化氧化及其去合金化效应
收稿日期: 2014-01-24
修回日期: 2014-04-01
网络出版日期: 2014-04-10
基金资助
国家自然科学基金项目(No. 21073045,No. 21273046)和上海市重点项目(No. 11JC140200,No. 08DZ2270500)资助
Electrocatalytic Oxidation of Formate on Pd-Cu/C - Effect of Dealloying Pretreatment
Received date: 2014-01-24
Revised date: 2014-04-01
Online published: 2014-04-10
蒋昆 , 王晔 , 林涛 , 蔡文斌 . Pd-Cu/C上甲酸根电催化氧化及其去合金化效应[J]. 电化学, 2014 , 20(4) : 343 -348 . DOI: 10.13208/j.electrochem.131173
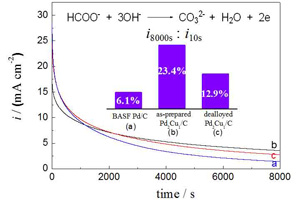
Key words: Pd-Cu alloy; electrocatalysts; formate ion; electrooxidation; dealloying effect
/
| 〈 |
|
〉 |