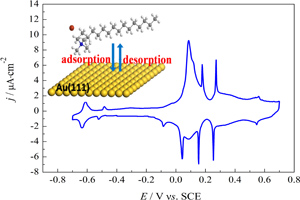
本文运用循环伏安方法研究十六烷基三甲基溴化铵(CTAB)在Au(111)电极上的吸附行为. 首次给出CTAB在Au(111)电极上的循环伏安曲线,其0.18 V、0.27 V有两对可逆的特征电流尖峰,均受扩散控制,且与卤素离子种类有关. 研究表明,烷基铵阳离子的吸脱附及吸附层相转变与Au(111)电极表面结构密切相关.
Adsorption of cetyltrimethylammonium bromide (CTAB) on Au(111) electrode was investigated by cyclic voltammetry (CV). The results demonstrate that the adsorption of CTAB on Au(111) yields particular CV features. Both the adsorption of CTA+ and the phase change of the surfactant film are surface structure selective. Two pairs of stable sharp peaks that depend on halide species appear upon the adsorption of cationic surfactant on Au(111) electrode. The relationship between the peak current density and the scan rate indicates that the electron transfer reaction was controlled by diffusion process. These CV features are observed for the first time, and reflect the specific interaction between CTAB and Au(111). The results are of significance in understanding the interaction of CTAB with Au surface for shape-control synthesis of Au nanoparticles.
[1] Haruta M, Yamada N, Kobayashi T, et al. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide[J]. Journal of Catalysis, 1989, 115(2): 301-309.
[2] Sau T K, Murphy C J. Room temperature, high-yield synthesis of multiple shapes of gold[J]. Journal of the American Chemical Society, 2004, 126(28): 8648-8649.
[3] Wang Z J, Yuan J H, Zhou M, et al. Synthesis, characterization and mechanism of cetyltrimethylammonium bromide bilayer-encapsulated gold nanosheets and nanocrystals[J]. Applied Surface Science, 2008, 254(20): 6289-6293.
[4] Zhang J, Langille M R, Personick M L, et al. Concave cubic gold nanocrystals with high-index facet[J]. Journal of the American Chemical Society, 2010, 132(40): 14012-14014.
[5] Lu D L, Tanaka K I. Au, Cu, Ag, Ni, and Pd particles grown in solution at different electrode potentials[J]. Journal of Physical Chemistry B, 1997, 101(20): 4030-4034.
[6] Jana N R, Gearheart L, Murphy C J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods[J]. Journal of Physical Chemistry B, 2001, 105(19): 4065-4067.
[7] Garg N, Scholl C, Mohanty A, et al. The role of bromide ions in seeding growth of Au nanorods[J]. Langmuir, 2010, 26(12): 10271-10276.
[8] Vivek J P, Burgess I J. Quaternary ammonium bromide surfactant adsorption on low-index surfaces of gold. 1. Au(111)[J]. Langmuir, 2012, 28(11): 5031-5039.
[9] Brosseau C L, Sheepwash E, Burgess I J, et al. Adsorption of N-decyl-N,N,N-trimethylammonium triflate (DeTATf), a cationic surfactant, on the Au(111) electrode surface[J]. Langmuir, 2007, 23(4): 1784-1791.
[10] Clavilier J, Faure R, Guinet G, et al. Preparation of mono-crystalline Pt microelectrodes and electrochemical study of the plane surfaces cut in the direction of the (111) and (110) planes[J]. Journal of Electroanalytical Chemistry, 1980, 107(1): 205-209.
[11] Harten U, Lahee A M, Toennies J P. Observation of a soliton reconstruction of Au(111) by high-resolution helium-atom diffraction[J]. Physical Review Letters, 1985, 54(24): 2619.
[12] Barth J V, Brune H, Ertl G. Scanning tunneling microscopy observations on the reconstructed Au(111) surface: Atomic structure, long-range superstructure, rotational domains, and surface defects[J]. Physical Review B, 1990, 42(15): 9307-9317.
[13] Ocko B M, Magnussen O M, Wang J X, et al. The structure and phase behavior of electrodeposited halides on single-crystal metal surfaces[J]. Physica B, 1996, 221(1-4): 238-244.
[14] Magnussen O M. Ordered anion adlayers on metal electrode surfaces[J]. Chemical Reviews, 2002, 102(3): 679-725.
[15] Shi Z C, Lipkowski J, Mirwald S T, et al. Electrochemical and second harmonic generation study of bromide adsorption at the Au(111) electrode surface[J]. Journal of the Chemical Society, Faraday Transactions, 1996, 92(20): 3737-3746.
[16] Tao N J, Lindsay S M. Kinetics of a potential induced 23 × to 1 × 1 transition of Au(111) studied by in situ scanning tunneling microscopy[J]. Surface Science Letters, 1992, 274(2): 546-553.
[17] Wang J, Davenport A J, et al. Surface charge-induced ordering of the Au(111) surface[J]. Science, 1992, 255(5050): 1416-1418.
[18] Kolb D M. Reconstruction phenomena at metal-electrolyte interfaces[J]. Progress in Surface Science, 1996, 51(2): 109-173.
[19] Gao X P, Hamelin A, Weaverb M J. Atomic relaxation at ordered electrode surfaces probed by scanning tunneling microscopy: Au(111) in aqueous solution compared with ultrahigh-vacuum environments[J]. Journal of Chemical Physics, 1991, 95(9): 6993-6996.
[20] Kawasaki H, Nishimura K, Arakawa R. Influence of the counterions of cetyltrimetylammonium salts on the surfactant adsorption onto gold surfaces and the formation of gold nanoparticles[J]. Journal of Physical Chemistry C, 2007, 111(6): 2683-2690.