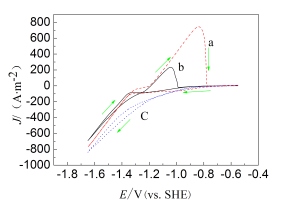
从槽液Mn2+浓度、电解时间和过电位关键因素研究电解初期过程,在三电极体系采用计时电量法探讨和优化工艺参数. 研究表明,在不锈钢表面,锰的初期电解析氢显著. 随时间延长,锰逐渐覆盖于不锈钢表面,析氢更困难,其电流效率随之提升,在高过电位区出现极限电流,反应受扩散控制. 在含0.02 g·L-1 SeO2溶液体系中,在40 g·L-1 Mn2+ + 120 g·L-1 (NH4)2SO4、过电位为0.151 V、槽液温度为40 oC、PH为6.6、时间为0.5 h的电解条件下,电流效率可达95.3%;在实用矿粉制液体系,效率也可达81.4%,较企业相同电解体系提高了15%.
This paper first studied the key factors affecting the initial manganese electrolysis process, including concentration of Mn2+, electrolysis time, overpotential, and then optimized these processing parameters with the chronocoulometry method under a three-electrode system. The results showed that, during the initial manganese electrolysis process, hydrogen evolution occurred more significantly on the stainless steel surface. As electrolysis process continued, manganese covered the stainless steel surface gradually, and hydrogen evolution reaction restrained which all contributed to the higher current efficiency. Besides, the limiting current density appeared at high overpotential region, and the reaction was controlled by diffusion process. In 0.02 g·L-1 SeO2 solution system with 40 g·L-1 Mn2+ and 120 g·L-1 (NH4)2SO4, electrolysis time of 0.5 h overpotential of 0.151 V, bath temperature of 40 oC, pH of 6.6, the current efficiency was achieved as high as 95.3%. And an efficiency of 81.4% was also realized in the industrialized electrolyte system, which was 15% higher as compared to the previous operation parameters under the same electrolyte system.