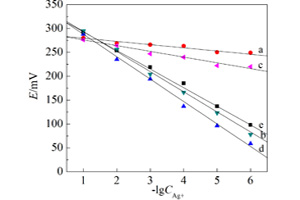
以硝酸银、凹凸棒石和硫代乙酰胺为原料制得硫化银/凹凸棒-Ag2S/ATT电极,并探讨了硫代乙酰胺配比、增塑剂用量、膜厚度以及溶液pH值等因素对电极性能的影响. 结果表明,新型银离子选择电极有较好的能斯特响应,其响应斜率48.0 mV·decade-1,Ag+浓度线性响应范围1.0×10-1 ~ 1.0×10-6 mol·L-1. 在pH = 2.0 ~ 8.0溶液中该电极电势可稳定72 h,对常见阳离子如Na+、Ca2+、Fe2+、Cu2+等呈现较强的抗干扰能力.
A novel silver ion selective electrode with its ionophore being a kind of organic/inorganic composite composed of silver sulfide and attapulgite has been prepared. Attapulgite is a type of natural inorganic polymer which has some properties similar to zeolite, such as a higher specific surface area and plenty of intercrystalline cavities. In this work, the silver ions were firstly adosrbed in the micropores of the attapulgite, then thioacetamidetiny was selected to mix with the prepared silver ions/ attapulgite composite. Owning to the existence of sulfur atoms in the structure of thioacetamide molecules, thioacetamide molecules can react with the silver ions/attapulgite composite so that tiny silver sulfide particles were formed within the pores of attapulgite. Experimental results showed that the prepared silver ion selective electrode with the silver sulfide/attapulgite composite as the ionophore exhibited the better linear responses toward silver ion measured and the quick response time (about 3 s). And effects of the concentration of thioacetamide on starting material, depth of electrode membrane, amount of plastic agent, pH of measured solution on the silver electrode performance had been investigated in this work. Experimental results also proved that the silver electrode displayed a proximate Nernst response with a linear slope of 48 mV·decade-1 in the silver concentrations from 1.0×10-1 to 1.0×10-6 mol·L-1. Within the range of pH = 2.0 ~ 8.0, the potential of the silver electrode had a better stability in 72 h. Also, the silver electrode showed better anti-interference in the measured solution containing some common metal ions like Na+, Ca2+, Fe2+ and Cu2+ etc.