

固态氧化物阴极过程的离子扩散模型及其Ta2O5熔盐电解验证
收稿日期: 2013-09-02
修回日期: 2013-11-06
网络出版日期: 2013-11-11
基金资助
国家自然科学基金项目(No. 21173161)及教育部新世纪人才计划项目(No. NCET-11-0397)资助
The Ionic Diffusion Model for the Solid Oxide Cathode and Its Verification by the Electrolysis of Ta2O5 in Molten CaCl2
Received date: 2013-09-02
Revised date: 2013-11-06
Online published: 2013-11-11
陈华林 , 王志勇 , 金先波 , 陈政 . 固态氧化物阴极过程的离子扩散模型及其Ta2O5熔盐电解验证[J]. 电化学, 2014 , 20(3) : 266 -271 . DOI: 10.13208/j.electrochem.130886
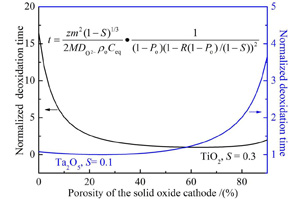
Key words: solid oxide; molten salt electrolysis; diffusion model; Ta2O5; Ta powder
/
| 〈 |
|
〉 |