

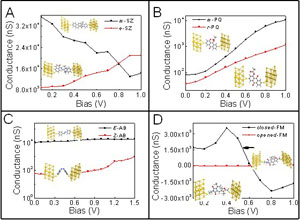
结构转变方式对光致变色分子开关输运性能影响的从头计算研究(英文)
收稿日期: 2013-08-08
修回日期: 2013-11-05
网络出版日期: 2013-11-10
基金资助
This work was supported by National Natural Science Foundation of China (Nos. 51071084, 21273113, 21121091 and 11204120) and National Key Technology R&D Program of China (No. 2012BAF03B05)
Effects of Conformational Transformations on Electronic Transport Properties of Optical Molecular Switches: An ab initio Study
Received date: 2013-08-08
Revised date: 2013-11-05
Online published: 2013-11-10
Supported by
This work was supported by National Natural Science Foundation of China (Nos. 51071084, 21273113, 21121091 and 11204120) and National Key Technology R&D Program of China (No. 2012BAF03B05)
贺园园 , 赵健伟 . 结构转变方式对光致变色分子开关输运性能影响的从头计算研究(英文)[J]. 电化学, 2014 , 20(3) : 243 -259 . DOI: 10.13208/j.electrochem.130881
/
| 〈 |
|
〉 |