

钠离子电池正极材料Na2MnPO4F的23Na MAS NMR谱研究
收稿日期: 2014-04-01
修回日期: 2014-04-14
网络出版日期: 2014-04-21
基金资助
973国家重点基础研究发展计划(No. 2011CB935903)和国家自然科学基金(No. 21233004、No. 21021002)项目资助
23Na MAS NMR Spectroscopic Study of Na2MnPO4F as Cathode Material for Sodium-Ion Battery
Received date: 2014-04-01
Revised date: 2014-04-14
Online published: 2014-04-21
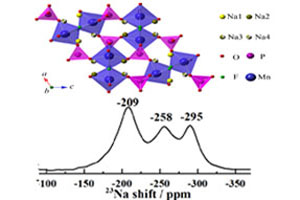
关键词: 钠离子电池; 正极材料; Na2MnPO4F; 23Na MAS NMR
侯旭 , 钟贵明 , 林晓琛 , 刘子庚 , 吴晓彪 , 杨勇 . 钠离子电池正极材料Na2MnPO4F的23Na MAS NMR谱研究[J]. 电化学, 2014 , 20(3) : 201 -205 . DOI: 10.13208/j.electrochem.140401

Key words: sodium ion batteries; cathode material; Na2MnPO4F; 23Na MAS NMR
/
| 〈 |
|
〉 |