

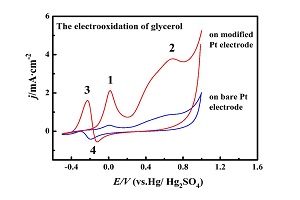
Nd-Fe-MoO42-氰桥混配聚合物修饰铂电极的丙三醇电催化氧化
收稿日期: 2013-07-23
修回日期: 2013-11-11
网络出版日期: 2014-04-17
基金资助
国家自然科学基金项目(No. 21174114)资助
Electrocatalytic Oxidation of Glycerol at the Platinum Electrode Modified with an Nd-Fe-MoO42- Cyano-Bridged Mixed Coordination Polymer
Received date: 2013-07-23
Revised date: 2013-11-11
Online published: 2014-04-17
马永钧 , 田玉秀 , 刘婧 , 周敏 , 丁静 , 金芝梅 , 王向梅 . Nd-Fe-MoO42-氰桥混配聚合物修饰铂电极的丙三醇电催化氧化[J]. 电化学, 2014 , 20(2) : 150 -155 . DOI: 10.13208/j.electrochem.130723

/
| 〈 |
|
〉 |