

基于氯化胆碱离子液体的铜电沉积研究(英文)
收稿日期: 2013-07-14
修回日期: 2013-08-19
网络出版日期: 2014-04-17
基金资助
The authors gratefully acknowledge the Ministry of Science and Technology, the People's Republic of Bangladesh for financial support of this work(Grand Reference No. MOSICT/PRC-24/2009-2010/BS-52/172).
Electrodeposition of Copper from a Choline Chloride based Ionic Liquid
Received date: 2013-07-14
Revised date: 2013-08-19
Online published: 2014-04-17
Supported by
The authors gratefully acknowledge the Ministry of Science and Technology, the People's Republic of Bangladesh for financial support of this work(Grand Reference No. MOSICT/PRC-24/2009-2010/BS-52/172).
M. Rostom Ali , Md. Ziaur Rahman , S. SankarSaha . 基于氯化胆碱离子液体的铜电沉积研究(英文)[J]. 电化学, 2014 , 20(2) : 139 -145 . DOI: 10.13208/j.electrochem.130714
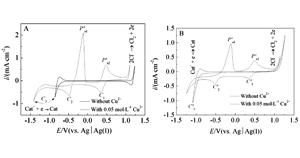
Key words: electrodeposition; ethaline; reline, copper; cyclic voltammetry
/
| 〈 |
|
〉 |