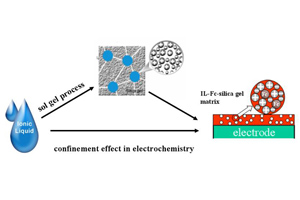
通过溶胶-凝胶法制备了硅胶包载咪唑类离子液体修饰电极,研究其与体相离子液体不同的伏安行为;另一方面,制备不同离子液体含量为15% ~ 28%的包载离子液体硅胶和涂覆离子液体硅胶,用电化学阻抗研究其在20 oC到80 oC下电导率的变化情况. 异常的电化学行为主要表现在:1)硅胶包载离子液体导致Fc/Fc+电对的半波电位正移63.5 ~ 200 mV;2)当离子液体限域于硅胶纳米孔道中时,离子液体的电化学稳定性变差;3)包载离子液体硅胶的电导率要比涂覆离子液体的电导率高29.6% ~ 136%. 由此推断,可能是由于离子液体充满硅胶孔腔和孔道从而形成了纳米网状的离子液体导电介质. 这些结果表明,硅胶包载离子液体不仅可以作为修饰电极的优良载体,而且也有助于理解离子液体限域于硅胶纳米孔道中的限域效应.
Imidazolium Ionic liquids (ILs) nano aggregates confined into nanopores of silica gel matrices (ILs-sg) modified electrodes were prepared by sol-gel process and characterized by cyclic voltammetry. Furthermore, the amounts of 15% ~ 28% ILs-sg and nano aggregates coated with ILs silica gel (ILs/sg) were prepared and their conductivities were evaluated by performing electrochemical AC impedance measurements from 20 oC to 80 oC. The abnormal results showed that, 1) comparing with the bulk IL, the confined IL had a positive shift effect of Fc/Fc+ redox potential; 2) when IL confined into a nanospace, the electrochemical stability became poor; 3) the ion conductivity of ILs-sg was 29.6% ~ 136% higher than that of ILs coated silica gel, which could be attributed to the formation of nano ionic liquid network electrolyte. These results not only illustrate that the ILs based silica gel could serve as an excellent support of modified electrode for electrochemical active substance, but also reveal that the findings are helpful to understand the electrochemical phenomena of ILs under a confinement environment.
[1] Márquez F, Martí V, Palomares E, et al. Observation of azo chromophore fluorescence and phosphorescence emissions from DBH by applying exclusively the orbital confinement effect in siliceous zeolites devoid of charge-balancing cations[J]. Journal of the American Chemical Society, 2002, 124(25): 7264-7265.
[2] Ravindra R, Zhao S, Gies H, et al. Protein encapsulation in mesoporous silicate: The effects of confinement on protein stability, hydration, and volumetric properties[J]. Journal of the American Chemical Society, 2004, 126(39): 12224-12225.
[3] Zhang J, Liu G, Jonas J. Effects of confinement on the glass transition temperature of molecular liquids[J]. The Journal of Physical Chemistry, 1992, 96(8): 3478-3480.
[4] Wallen S, Nikiel L, Yi J, et al. Confinement effects on the dynamics of liquid carbon disulfide[J]. The Journal of Physical Chemistry, 1995, 99(42):15421-15427.
[5] Xu S, Ballard L, Kim Y J, et al. Dynamic structure of methylcyclohexane and perfluoromethylcyclohexane liquids in confinement and in bulk[J]. The Journal of Physical Chemistry, 1995, 99(16): 5787-5792.
[6] Dosseh G, Xia Y, Alba-Simionesco C. Cyclohexane and benzene confined in MCM-41 and SBA-15: confinement effects on freezing and melting[J]. The Journal of Physical Chemistry B, 2003, 107(26): 6445-6453.
[7] Zhang J, Liu G, Jonas J, et al. Effects of confinement on the glass transition temperature of molecular liquids[J]. The Journal of Physical Chemistry, 1992, 96(8): 3478-3480.
[8] Néouze M, Bideau J L, Leroux F, et al. A route to heat resistant solid membranes with performances of liquid electrolytes[J]. Chemical Communications, 2005, 8: 1082-1084.
[9] Néouze M, Bideau J L, Gaveau P, et al. Ionogels, new materials arising from the confinement of ionic liquids within silica-derived networks[J]. Chemistry of Materials, 2006, 18(17): 3931-3936.
[10] Wasserscheid P, Welton T. Ionic liquids in synthesis[M]. Weinheim: Wiley-VCH, 2003.
[11] Ohno H. Electrochemical aspects of ionic liquids[M]. Weinheim: Wiley-VCH, 2005.
[12] Shi F, Deng Y. Abnormal FT-IR and FTRaman spectra of ionic liquids confined in nano-porous silica gel[J]. Spectrochimica Acta Part A, 2005, 62(1/3): 239-244.
[13] Zhang J, Zhang Q, Shi F, et al. Greatly enhanced fluorescence of dicyanamide anion based ionic liquids confined into mesoporous silica gel[J]. Chemical Physics Letters, 2008, 461(4/6): 229-234.
[14] Zhang J, Zhang Q, Li X, et al. Nanocomposites of ionic liquids confined in mesoporous silica gels: Preparation, characterization and performance[J]. Physical Chemistry Chemical Physics, 2010, 12(8): 1971-1981.
[15] Singh M P, Singh R K, Chandra S. Properties of ionic liquid confined in porous silica matrix[J]. ChemPhysChem, 2010, 11(9): 2036-2043.
[16] Kanakubo M, Hiejima Y, Minami K, et al. Melting point depression of ionic liquids confined in nanospaces[J]. Chemical Communications, 2006, 17: 1828-1830.
[17] Gobel R, Hesemann P, Weber J, et al. Surprisingly high, bulk liquid-like mobility of silica-confined ionic liquids[J]. Physical Chemistry Chemical Physics, 2009, 11(19): 3653-3662.
[18] Chen S, Wu G, Sha M, et al. Transition of ionic liquid [bmim][PF6] from liquid to high-melting-point crystal when confined in multiwalled carbon nanotubes[J]. Journal of the American Chemical Society, 2007, 129(9): 2416-2417.
[19] Bonh?te P, Dias A P, Papageorgiou N, et al. Hydrophobic, highly conductive ambient-temperature molten salts[J]. Inorganic Chemistry, 1996, 35(5): 1168-1178.
[20] Wilkes J S, Zaworotko M J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids[J]. Journal of the Chemical Society, Chemical Communications, 1992, 965-967.
[21] Egashira M, Yamamoto Y, Fukutake T, et al. A novel method for preparation of imidazolium tetrafluoroborate ionic liquids[J]. Journal of Fluorine Chemistry, 2006, 127(9): 1261-1264.
[22] Wang Q, Lu G, Yang B. Myoglobin/sol-gel film modified electrode: Direct electrochemistry and electrochemical catalysis[J]. Langmuir, 2004, 20(4): 1342-1347.
[23] Shustak G, Marx S, Turyan, I, et al. Application of sol-gel technology for electroanalytical sensing[J]. Electroanalysis, 2003, 15(5/6): 398-480.
[24] Kroon M C, Buijs W, Petersa C J, et al. Decomposition of ionic liquids in electrochemical processing[J]. Green Chemistry, 2006, 8(3): 241-245.



