

Pt/H-TiO2催化剂制备及其甲醇电催化氧化性能
收稿日期: 2013-04-17
修回日期: 2013-05-16
网络出版日期: 2014-04-17
基金资助
国家自然科学基金项目(No. 20933004)资助
Preparation of Pt/H-TiO2 Catalyst with Improved Catalytic Performance for Methanol Electrooxidation
Received date: 2013-04-17
Revised date: 2013-05-16
Online published: 2014-04-17
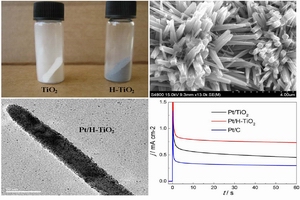
韩金 , 周志有 , 汪强 , 吕妙强 , 陈驰 , 孙世刚 . Pt/H-TiO2催化剂制备及其甲醇电催化氧化性能[J]. 电化学, 2014 , 20(2) : 110 -115 . DOI: 10.13208/j.electrochem.130418

/
| 〈 |
|
〉 |