

pH/Cl-复合探针技术对钢筋混凝土电化学除氯的原位检测
收稿日期: 2013-05-31
修回日期: 2013-09-23
网络出版日期: 2014-04-17
基金资助
国家自然科学基金项目(No. 21203158, No. 21021002)资助
In Situ Detection on Electrochemical Chloride Removal of Reinforcement in Concrete by Combined pH/Cl- Probes
Received date: 2013-05-31
Revised date: 2013-09-23
Online published: 2014-04-17
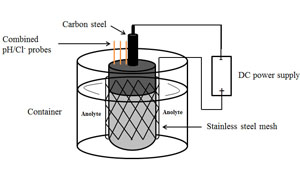
关键词: pH/Cl-复合探针; 电化学除氯; 钢筋混凝土; 腐蚀
王静静 , 董士刚 , 张小娟 , 吕虹玮 , 伍运昌 , 杜荣归 , 林昌健 . pH/Cl-复合探针技术对钢筋混凝土电化学除氯的原位检测[J]. 电化学, 2014 , 20(2) : 95 -100 . DOI: 10.13208/j.electrochem.130531

/
| 〈 |
|
〉 |