

乙烯硫脲-格氏试剂/THF电解液镁沉积-溶出性能
收稿日期: 2012-09-30
修回日期: 2013-01-21
网络出版日期: 2014-02-24
基金资助
国家自然科学基金项目(No. 21273147,No. 51035005)和上海市科委项目(No. 11JC1405700)资助
Magnesium Deposition-Dissolution Performance of Ethylenethiourea-Grignard Reagent/THF Electrolytes
Received date: 2012-09-30
Revised date: 2013-01-21
Online published: 2014-02-24
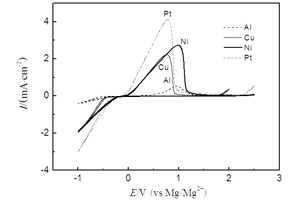
卞沛文 , 努丽燕娜 , 陈强 , 杨军 , 王久林 . 乙烯硫脲-格氏试剂/THF电解液镁沉积-溶出性能[J]. 电化学, 2014 , 20(1) : 73 -79 . DOI: 10.13208/j.electrochem.120930

/
| 〈 |
|
〉 |