

中温固体氧化物燃料电池LaNi0.6Fe0.4O3-δ-Gd0.2Ce0.8O2梯度复合阴极制备及交流阻抗性能
收稿日期: 2012-12-06
修回日期: 2013-02-21
网络出版日期: 2014-02-24
基金资助
国家自然科学基金项目(No. 51201098)资助
Fabrication and Impedance Performance of Gradient LaNi0.6Fe0.4O3-δ-Gd0.2Ce0.8O2 Composite Cathodes for Intermediate Temperature Solid Oxide Fuel Cell
Received date: 2012-12-06
Revised date: 2013-02-21
Online published: 2014-02-24
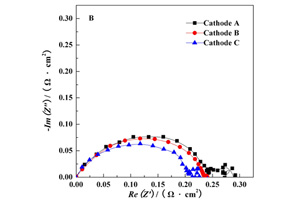
李扬 , 黄波 , 袁梦 , 张志秋 , 刘宗尧 , 唐旭晨 , 朱新坚 . 中温固体氧化物燃料电池LaNi0.6Fe0.4O3-δ-Gd0.2Ce0.8O2梯度复合阴极制备及交流阻抗性能[J]. 电化学, 2014 , 20(1) : 45 -50 . DOI: 10.13208/j.electrochem.121206

/
| 〈 |
|
〉 |