

酸浸蚀Al-Si合金制备锂离子电池高性能多孔硅负极材料
Preparation of High Performance Porous Silicon Powders by Etching Al-Si Alloy in Acid Solution for Lithium Ion Battery
Received date: 2013-04-26
Revised date: 2013-07-01
Online published: 2014-02-24
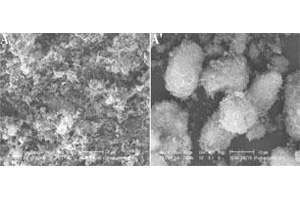
郝世吉 , 李纯莉 , 朱凱 , 张平 , 江志裕 . 酸浸蚀Al-Si合金制备锂离子电池高性能多孔硅负极材料[J]. 电化学, 2014 , 20(1) : 1 -4 . DOI: 10.13208/j.electrochem.130426

/
| 〈 |
|
〉 |