电化学(中英文) ›› 2024, Vol. 30 ›› Issue (2): 2303141. doi: 10.13208/j.electrochem.2303141
所属专题: “教程”专题文章
王南, 黄秋安*( ), 李伟恒*(
), 李伟恒*( ), 白玉轩, 张久俊*(
), 白玉轩, 张久俊*( )
)
收稿日期:2023-03-22
修回日期:2023-05-31
接受日期:2023-06-05
发布日期:2023-06-08
出版日期:2024-02-28
通讯作者:
* 黄秋安,Tel:(86)18827068633,E-mail:qiuan_huang@shu.edu.cn;
李伟恒,Tel:(86)19946238582,E-mail:wh_li@shu.edu.cn;
张久俊,Tel:(86)13918734136,E-mail:jiujun.zhang@i.shu.edu.cn基金资助:
Nan Wang, Qiu-An Huang*( ), Weiheng Li*(
), Weiheng Li*( ), Yuxuan Bai, Jiujun Zhang*(
), Yuxuan Bai, Jiujun Zhang*( )
)
Received:2023-03-22
Revised:2023-05-31
Accepted:2023-06-05
Online:2023-06-08
Published:2024-02-28
摘要:
近年来,联合时频分析再次成为研究热点。超级电容器功率密度高和寿命长,但为了优化平衡功率密度和能量密度,需考虑两个关键因素:(1)多孔基质的比表面积;(2)孔内空间电解质可抵达性。本文采用联合时频分析方法,研究孔内电荷穿透深度及电流空间分布。具体开展了如下工作:(i)在复正弦电流激励下,推导单孔的时域响应和频域响应解析解,由此定义了描述电荷扩散行为的时频特征。(ii)采用联合时频方法,分析了内部参数和外部参数对孔内电荷穿透率的影响,揭示了孔内电荷有限扩散和无限扩散之间的演变规律。(iii)基于穿透率临界值,定义了孔内部参数的临界值,由此判断孔内电荷半无限扩散和有限扩散。本文提出联合时频分析方法,实现了多孔电极中复杂物理化学过程的信息融合,联合时频分析最终殊途同归,并提高诊断可靠性。
王南, 黄秋安, 李伟恒, 白玉轩, 张久俊. 联合时频分析:以单孔中电荷穿透深度和电流空间分布为例[J]. 电化学(中英文), 2024, 30(2): 2303141.
Nan Wang, Qiu-An Huang, Weiheng Li, Yuxuan Bai, Jiujun Zhang. Joint Time-Frequency Analysis: taking Charge Penetration Depth and Current Spatial Distribution in the Single Pore as An Example[J]. Journal of Electrochemistry, 2024, 30(2): 2303141.
表1.
单孔参数及观测频率的默认值
| Parameter | Default value | Unit | Reference |
|---|---|---|---|
| Interface capacitance per unit area Cdl | C0=1 | F·m-2 | [36,37] |
| Liquid electrolyte conductivity σ | σ0=8×10-2 | S·m-1 | [16,38] |
| Pore length L | L0=6×10-7 | m | [22,39] |
| Pore diameter d | d0=36×10-9 | m | [22,40] |
| Observed frequency fO | 103 | Hz | [41,42] |
| Current density ja | ja0=3×103 | A·m-2 | [43-49] |
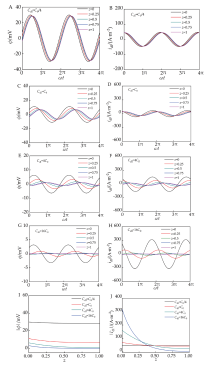
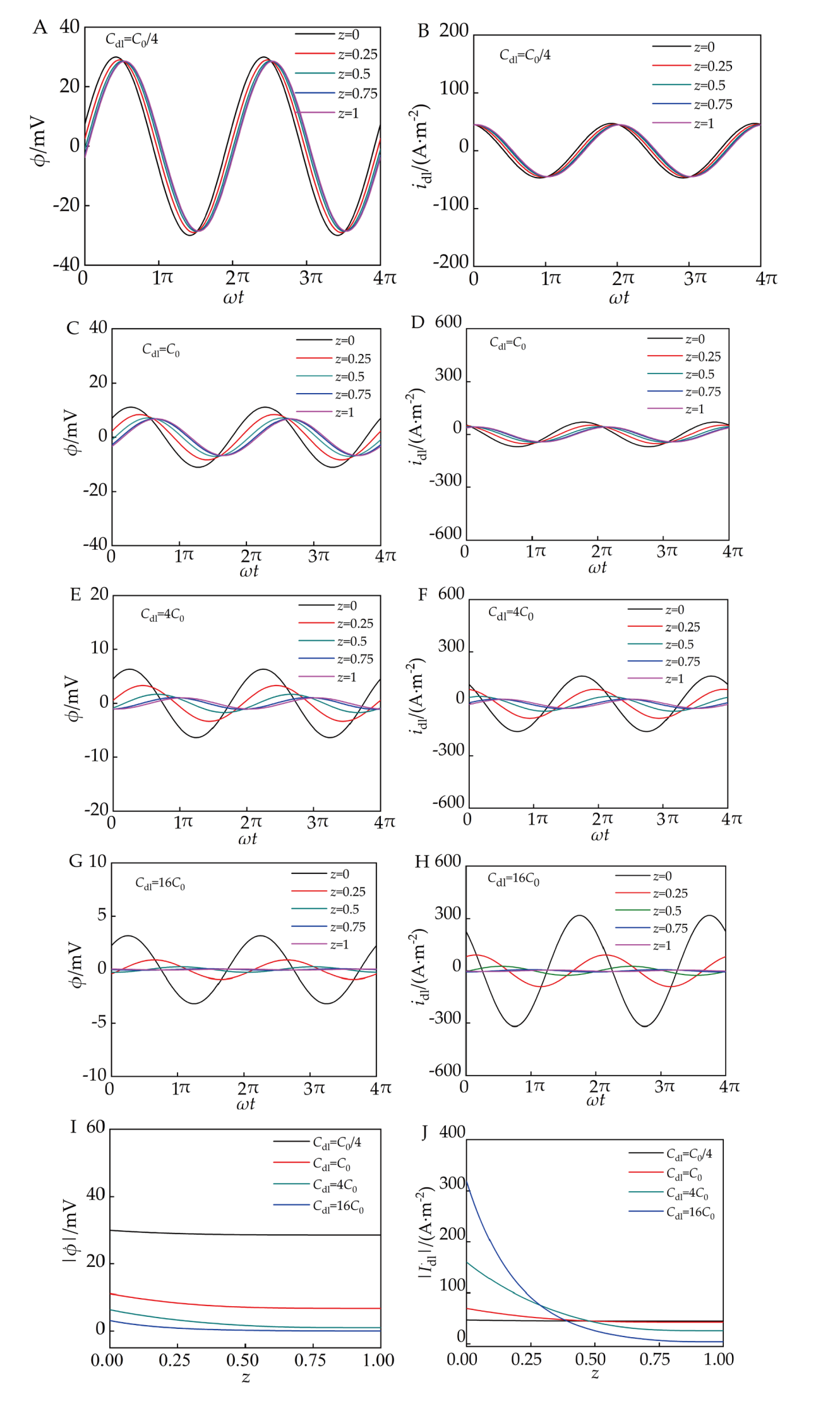
图10.
在1.0 kHz复正弦电流激励下,ϕ(z,t)和idl(z,t)随Cdl和z的时域响应。(A)Cdl=C0/4 时ϕ(z,t);(B)Cdl=C0/4时idl(z,t);(C)Cdl=C0 时ϕ(z,t);(D)Cdl=C0 时idl(z,t);(E)Cdl=4C0时ϕ(z,t);(F)Cdl=4C0时idl(z,t);(G)Cdl=16C0时ϕ(z,t);(H)Cdl=16C0时idl(z,t);Cdl=C0/4,C0,4 C0和16 C0时(I) ϕ ˙ - z和(J) I ˙ d l - z. Under a complex sinusoidal current excitation at f=1.0 kHz, the time-domain responses of ϕ(z,t) and idl(z,t) with respect to C d l and z. (A) ϕ(z,t) at Cdl=C0/4; (B) idl(z,t) at Cdl=C0/4; (C) ϕ(z,t) at Cdl=C0; (D) idl(z,t) at Cdl=C0; (E) ϕ(z,t) at Cdl=4C0 ; (F) idl(z,t) at Cdl=4C0; (G) ϕ(z,t) at Cdl=16C0; (H) idl(z,t) at Cdl=16C0; At Cdl=C0/4, C0, 4 C0, and 16 C0; (I) ϕ ˙ - z 和 (J) I ˙ d l - z.
| [1] |
González A, Goikolea E, Barrena J A, Mysyk R. Review on supercapacitors: technologies and materials[J]. Renew. Sust. Energ. Rev., 2016, 58: 1189-1206.
doi: 10.1016/j.rser.2015.12.249 URL |
| [2] |
Raza W, Ali F, Raza N, Luo Y, Kim K H, Yang J, Kumar S, Mehmood A, Kwon E E. Recent advancements in supercapacitor technology[J]. Nano Energy, 2018, 52: 441-473.
doi: 10.1016/j.nanoen.2018.08.013 URL |
| [3] |
Salanne M, Rotenberg B, Naoi K, Kaneko K, Taberna P L, Grey C P, Dunn B, Simon P. Efficient storage mechanisms for building better supercapacitors[J]. Nat. Energy, 2016, 1(6): 16070.
doi: 10.1038/nenergy.2016.70 |
| [4] |
Jadhav P R, Suryawanshi M P, Dalavi D S, Patil D S, Jo E A, Kolekar S S, Wali A A, Karanjkar M M, Kim J Hyeok, Patil P S. Design and electro-synthesis of 3-D nanofibers of MnO2 thin films and their application in high performance supercapacitor[J]. Electrochim. Acta, 2015, 176: 523-532.
doi: 10.1016/j.electacta.2015.07.002 URL |
| [5] |
Sonai Muthu N, Gopalan M. Polyethylene glycol-assisted growth of Ni3S4 closely packed nanosheets on Ni-foam for enhanced supercapacitor device[J]. J. Solid State Electrochem., 2019, 23(10): 2937-2950.
doi: 10.1007/s10008-019-04392-5 |
| [6] |
Wang H, Forse A C, Griffin J M, Trease N M, Trognko L, Taberna P L, Simon P, Grey C P. In situ NMR spectroscopy of supercapacitors: Insight into the charge storage mechanism[J]. J. Am. Chem. Soc., 2013, 135(50): 18968-18980.
doi: 10.1021/ja410287s pmid: 24274637 |
| [7] |
Chen J, Lee P S. Electrochemical supercapacitors: From mechanism understanding to multifunctional applications[J]. Adv. Energy Mater., 2021, 11(6): 2003311.
doi: 10.1002/aenm.v11.6 URL |
| [8] |
Kurzweil P, Chwistek M. Electrochemical stability of organic electrolytes in supercapacitors: Spectroscopy and gas analysis of decomposition products[J]. J. Power Sources, 2008, 176(2): 555-567.
doi: 10.1016/j.jpowsour.2007.08.070 URL |
| [9] | Lin Z, Taberna P L, Simon P. Advanced analytical techniques to characterize materials for electrochemical capacitors[J]. Curr. Opin. Electrochem., 2018, 9: 18-25. |
| [10] | Ciucci F. Modeling electrochemical impedance spectroscopy[J]. Curr. Opin. Electrochem., 2019, 13: 132-139. |
| [11] |
Bi S, Banda H, Chen M, Niu L, Chen M Y, Wu T Z, Wang J S, Wang R X, Feng J M, Chen T Y, Dincă M, Kornyshev A A, Feng G. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes[J]. Nat. Mater., 2020, 19(5): 552-558.
doi: 10.1038/s41563-019-0598-7 pmid: 32015536 |
| [12] |
Zhang L Y, Shi D W, Liu T, Jaroniec M, Yu J G. Nickel-based materials for supercapacitors[J]. Mater. Today, 2019, 25: 35-65.
doi: 10.1016/j.mattod.2018.11.002 |
| [13] |
Lazanas A Ch, Prodromidis M I. Electrochemical impedance spectroscopy─A tutorial[J]. ACS Meas. Sci. Au, 2023, 3 (3): 162-193.
doi: 10.1021/acsmeasuresciau.2c00070 URL |
| [14] |
Allagui A, Fouda M E, Elwakil A, Psychalinos C. Time-domain response of supercapacitors using their impedance parameters and fourier series decomposition of the excitation signal[J]. J. Electroanal. Chem., 2023, 947: 117751.
doi: 10.1016/j.jelechem.2023.117751 URL |
| [15] | Barsoukov E, Macdonald R J. Impedance spectroscopy: Theory, experiment, and applications[M]. USA: Wiley-Interscience, 2005. |
| [16] | Lasia A. Electrochemical impedance spectroscopy and its applications[M]. New York: Springer, 2014. |
| [17] | Wang X X, Zhou Z Z, Shan Q, Zhang Z M, Huang J, Liu Y W, Chen S L. Porous-electrode theory of lithium ion battery: old paradigm and new challenge[J]. J. Electrochem., 2020, 26(5): 596-606. |
| [18] | Ji W X, Wang G W, Wang Q, Bai L J, Qu D Y. Porous electrodes in electrochemical energy storage systems[J]. J. Electrochem., 2020, 26(5): 576-595. |
| [19] |
Li X, Huang Q A, Li W H, Bai Y X, Wang J, Liu Yang, Zhao Y F, Wang J, Zhang J J. Fundamentals of electrochemical impedance spectroscopy for macrohomogeneous porous electrodes[J]. J. Electrochem., 2021, 27(5): 467-497.
doi: 10.13208/j.electrochem.201126 |
| [20] | De Levies R. On porous electrodes in electrolyte solutions[J]. Elecrrochim. Acta, 1963, 8(10): 751-780. |
| [21] |
De Levies R. On porous electrodes in electrolyte solutions-IV[J]. Electrochim. Acta, 1964, 9(9): 1231-1245.
doi: 10.1016/0013-4686(64)85015-5 URL |
| [22] |
Candy J P, Fouilloux P, Keddam M, Takenouti H. The characterization of porous electrodes by impedance measurements[J]. Electrochim. Acta, 1981, 26(8): 1029-1034.
doi: 10.1016/0013-4686(81)85072-4 URL |
| [23] |
Keiser H, Beccu K D, Gutjahr M A. Abschätzung der porenstruktur poröser elektroden aus impedanzmessungen[J]. Electrochim. Acta, 1976, 21(8): 539-543.
doi: 10.1016/0013-4686(76)85147-X URL |
| [24] |
Linneen N, Delnick F, Islam S Z, Deshmane V G, Bhave R. Application of the macrohomogeneous line model for the characterization of carbon aerogel electrodes in capacitive deionization[J]. Electrochim. Acta, 2019, 301: 1-7.
doi: 10.1016/j.electacta.2019.01.123 URL |
| [25] |
Paasch G, Micka K, Gersdorf P. Theory of the electrochemical impedance of macrohomogeneous porous electrodes[J]. Electrochim. Acta, 1993, 38(18): 2653-2662.
doi: 10.1016/0013-4686(93)85083-B URL |
| [26] |
Song H K, Hwang H Y, Lee K H, Dao L H. The effect of pore size distribution on the frequency dispersion of porous electrodes[J]. Electrochim. Acta, 2000, 45(14): 2241-2257.
doi: 10.1016/S0013-4686(99)00436-3 URL |
| [27] |
Yoo H D, Jang J H, Ryu J H, Park Y, Oh S M. Impedance analysis of porous carbon electrodes to predict rate capability of electric double-layer capacitors[J]. J. Power Sources, 2014, 267: 411-420.
doi: 10.1016/j.jpowsour.2014.05.058 URL |
| [28] |
Li W H, Huang Q A, Li Y, Bai Y, Wang N, Wang J, Hu Y M, Zhao Y F, Li X F, Zhang J J. Capacitive energy storage from single pore to porous electrode identified by frequency response analysis[J]. J. Energy Chem., 2023, 77: 384-405.
doi: 10.1016/j.jechem.2022.10.017 |
| [29] | El Brouji H, Vinassa J M, Briat O, Bertrand N, Woirgard E. Ultracapacitors self discharge modelling using a physical description of porous electrode impedance[C]. El Brouji H, IEEE Vehicle Power and Propulsion Conference, China: IEEE, 2008, 1-6. |
| [30] |
Meyers J P, Doyle M, Darling R M, Newman J. The impedance response of a porous electrode composed of intercalation particles[J]. J. Electrochem. Soc., 2000, 147(8): 2930.
doi: 10.1149/1.1393627 URL |
| [31] |
Siroma Z, Fujiwara N, Yamazaki S, Asahi M, Nagai T, Ioroi T. Mathematical solutions of comprehensive variations of a transmission-line model of the theoretical impedance of porous electrodes[J]. Electrochim. Acta, 2015, 160: 313-322.
doi: 10.1016/j.electacta.2015.02.065 URL |
| [32] | Zhuang Q C, Yang Z, Zhang L, Cui Y H. Research progress on diagnosis of electrochemical impedance spectroscopy in lithium ion batteries[J]. Prog. Chem., 2020, 32(06): 761-791. |
| [33] |
Johnson A M, Newman J. Desalting by means of porous carbon electrodes[J]. J. Electrochem. Soc., 1971, 118(3): 510-517.
doi: 10.1149/1.2408094 URL |
| [34] |
Gomadam P M, Weidner J W, Zawodzinski T A, Saab A P. Theoretical analysis for obtaining physical properties of composite electrodes[J]. J. Electrochem. Soc., 2003, 150(8): E371.
doi: 10.1149/1.1586301 URL |
| [35] |
Huang Q A, Li W H, Tang Z P, Zhang F Z, Li A J, Zhang J J. Fundamentals of electrochemical impedance spectroscopy[J]. Chin. J. Nat., 2020, 42(1): 12-26.
doi: 10.3969/j.issn.0253-9608.2020.01.002 |
| [36] |
Nguyen T T, Demortière A, Fleutot B, Delobel B, Delacourt C, Cooper S J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead[J]. npj Comput. Mater., 2020, 6(1): 123.
doi: 10.1038/s41524-020-00386-4 |
| [37] |
Honda K, Rao T N, Tryk D A, Fujishima A, Watanabe M, Yasui K, Masuda H. Impedance characteristics of the nanoporous honeycomb diamond electrodes for electrical double-layer capacitor applications[J]. J. Electrochem. Soc., 2001, 148(7): A668.
doi: 10.1149/1.1373450 URL |
| [38] |
Siroma Z, Sato T, Takeuchi T, Nagai R, Ota A, Ioroi T. AC impedance analysis of ionic and electronic conductivities in electrode mixture layers for an all-solid-state lithium-ion battery[J]. J. Power Sources, 2016, 316: 215-223.
doi: 10.1016/j.jpowsour.2016.03.059 URL |
| [39] |
Bisquert J, Garcia-Belmonte G, Fabregat-Santiago F, Compte A. Anomalous transport effects in the impedance of porous film electrodes[J]. Electrochem. Commun., 1999, 1(9): 429-435.
doi: 10.1016/S1388-2481(99)00084-3 URL |
| [40] |
Wang D W, Li F, Liu M, Lu G Q, Cheng H M. Mesopore-aspect-ratio dependence of ion transport in rodtype ordered mesoporous carbon[J]. J. Phys. Chem. C, 2008, 112(26): 9950-9955.
doi: 10.1021/jp800173z URL |
| [41] |
Huang Q A, Li Y, Tsay K C, Sun C, Changping Yang, Zhang L, Zhang J. Multi-scale impedance model for supercapacitor porous electrodes: Theoretical prediction and experimental validation[J]. J. Power Sources, 2018, 400: 69-86.
doi: 10.1016/j.jpowsour.2018.07.108 URL |
| [42] |
Musiani M, Orazem M, Tribollet B, Vivier V. Impedance of blocking electrodes having parallel cylindrical pores with distributed radii[J]. Electrochim. Acta, 2011, 56(23): 8014-8022.
doi: 10.1016/j.electacta.2010.12.004 URL |
| [43] | Huang J, Zhang J. Theory of impedance response of porous electrodes: simplifications, inhomogeneities, non-stationarities and applications[J]. J. Electrochem. Soc., 2016, 163(9): A1983-A2000. |
| [44] |
Yuan X, Wang H, Colinsun J, Zhang J. AC impedance technique in PEM fuel cell diagnosis—A review[J]. Int. J. Hydrogen Energy, 2007, 32(17): 4365-4380.
doi: 10.1016/j.ijhydene.2007.05.036 URL |
| [45] |
Tang Y, Zhang J, Song C, Liu H, Zhang J, Wang H, Mackinnon S, Peckham T, Li J, Mcdermid S, Kozak P. Temperature dependent performance and in situ AC impedance of high-temperature PEM fuel cells using the Nafion-112 membrane[J]. J. Electrochem. Soc., 2006, 153(11): A2036.
doi: 10.1149/1.2337008 URL |
| [46] |
Wagner N, Gülzow E. Change of electrochemical impedance spectra (EIS) with time during CO-poisoning of the Pt-anode in a membrane fuel cell[J]. J. Power Sources, 2004, 127(1-2): 341-347.
doi: 10.1016/j.jpowsour.2003.09.031 URL |
| [47] |
Brett D J L, Atkins S, Brandon N P, Vesovic V, Vasileiadis N, Kucernak A. Localized impedance measurements along a single channel of a solid polymer fuel cell[J]. Electrochem. Solid-State Lett., 2003, 6(4): A63.
doi: 10.1149/1.1557034 URL |
| [48] |
Iranzo A, Muñoz M, Pino Fco J, Rosa F. Non-dimensional analysis of PEM fuel cell phenomena by means of AC impedance measurements[J]. J. Power Sources, 2011, 196(9): 4264-4269.
doi: 10.1016/j.jpowsour.2010.11.004 URL |
| [49] |
Jang J H, Oh S M. Complex capacitance analysis of porous carbon electrodes for electric double-layer capacitors[J]. J. Electrochem. Soc., 2004, 151(4): A571.
doi: 10.1149/1.1647572 URL |
| [50] | Li Y, Yang W M, Huang Q A, Li W H, Li X F, Zhang J J. Simulation of warburg impedance spectra under finite diffusion boundary conditions for porous energy electrode materials[J]. J. Xi'an Univ. Technol., 2019, 35(2): 138-146. |
| [51] |
Jang J H, Yoon S, Ka B H, Jung Y H, Oh S M. Complex capacitance analysis on leakage current appearing in electric double-layer capacitor carbon electrode[J]. J. Electrochem. Soc., 2005, 152(7): A1418.
doi: 10.1149/1.1931469 URL |
| [52] |
Taberna P L, Simon P, Fauvarque J F. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors[J]. J. Electrochem. Soc., 2003, 150(3): A292.
doi: 10.1149/1.1543948 URL |
| [53] |
Itagaki M, Suzuki S, Shitanda I, Watanabe K. Electrochemical impedance and complex capacitance to interpret electrochemical capacitor[J]. Electrochemistry, 2007, 75(8): 649-655.
doi: 10.5796/electrochemistry.75.649 URL |
| [54] |
Huang J, Gao Y, Luo J, Wang S, Li C, Chen S, Zhang J. Editors’ Choice-Review-Impedance response of porous electrodes: Theoretical framework, physical models and applications[J]. J. Electrochem. Soc., 2020, 167(16): 166503.
doi: 10.1149/1945-7111/abc655 |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||